 |
 |
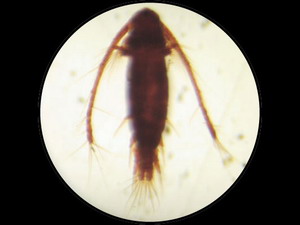
Acartia tonsa - dorsal view (magnification 80). |
Acartia tonsa - lateral view (magnification 80). INTERNET: http://homepage.mac.com/a.shiroza/plankton/bwttf/ acartia_lateral80_e.html |
Acartia tonsa (Dana, 1848)
| Subtypus | Invertebrata | |
| Order | Copepoda | |
| Suborder | Calanoida | |
| Family | Acartiidae | |
| Genus | Acartia (Dana, 1846) | |
| Species | Acartia tonsa (Dana, 1848) |
 |
 |
Acartia tonsa - dorsal view (magnification 80). |
Acartia tonsa - lateral view (magnification 80). INTERNET: http://homepage.mac.com/a.shiroza/plankton/bwttf/ acartia_lateral80_e.html |